Alkanes are saturated open chain hydrocarbons containing carbon – carbon single bonds.
Methane (CH4) is the first member of this family. Methane is a gas found in coal mines and marshy places.
The lighter ones are gases and used as fuels. The middle ones (7 Carbons to 12 Carbons) are liquids used in petrol (gasoline). The higher ones are waxy solids. Candle wax is a mixture of alkanes.
Alkanes are saturated which means they contain the maximum number of hydrogens per carbon and no double or triple bonds.

If you replace one hydrogen atom of methane by carbon and join the required number of hydrogens to satisfy the tetravalence of the other carbon atom, what do you get? You get C2H6. (Ethane)
Thus you can consider C2H6 as derived from CH4 by replacing one hydrogen atom by -CH3 group.
Go on constructing alkanes by doing this theoretical exercise i.e., replacing hydrogen atom by –CH3 group.
The next molecules will be C3H8, C4H10…

These hydrocarbons are inert under normal conditions as they do not react with acids, bases and other reagents. Hence, they were earlier known as paraffins (latin : parum, little; affinis, affinity).
Can you think of the general formula for alkane family or homologous series? If we examine the formula of different alkanes we find that the general formula for alkanes is CnH2n+2. It represents any particular homologue when n is given appropriate value.
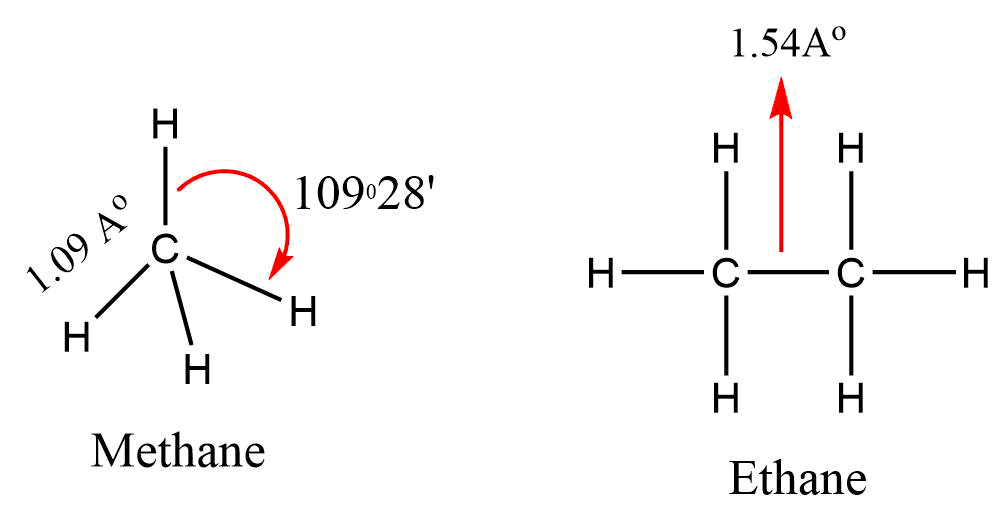
According to VSEPR Theory methane has a tetrahedral structure, in which carbon atom lies at the centre and the four hydrogen atoms lie at the four corners of a regular tetrahedron. All H-C-H bond angles are of 109.5°.
In alkanes, tetrahedra are joined together in which C-C and C-H bond lengths are 154 pm and 112 pm respectively.
C–C and C–H σ bonds are formed by head-on overlapping of sp3 hybrid orbitals of carbon and 1s orbitals of hydrogen atoms.
Nomenclature and Isomerism
First three alkanes – Methane, Ethane and Propane have only one type of structure i.e. continuous
chain but higher alkanes can have more than one structure.


Let us write structures for C4H10. Four carbon atoms of C4H10 can be joined either in a continuous chain or with a branched chain in the following two ways :


In how many ways, you can join five carbon atoms and twelve hydrogen atoms of C5H12?
They can be arranged in three ways as shown in structures III–V



Structures I and II possess same molecular formula but differ in their boiling points and other properties. Similarly structures III, IV and V possess the same molecular formula but have different properties.
Structures I and II are isomers of butane, whereas structures III, IV and V are isomers of pentane.
Since difference in properties is due to difference in their structures, they are known as structural isomers.
It is also clear that structures I and III have continuous chain of carbon atoms but structures II, IV and V have a branched chain. Such structural isomers which differ in chain of carbon atoms are known as chain isomers.
Thus, you have seen that C4H10 and C5H12 have two and three chain isomers respectively.

Based upon the number of carbon atoms attached to a carbon atom, the carbon atom is
termed as primary (1°), secondary (2°), tertiary (3°) or quaternary (4°).
- Carbon atom attached to no other carbon atom as in methane or to only one carbon atom as in ethane is called primary carbon atom. Terminal carbon atoms are always primary.
- Carbon atom attached to two carbon atoms is known as secondary.
- Tertiary carbon is attached to three carbon atoms and neo or quaternary carbon is attached to four carbon atoms.
C6H14 has got five isomers and C7H16 has nine. As many as 75 isomers are possible for C10H22.