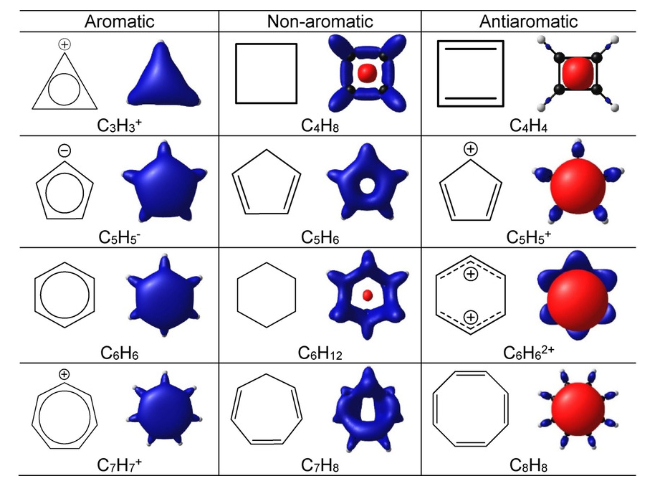
Benzene was considered as parent ‘aromatic’ compound. Now, the name is applied to all the ring systems whether or not having benzene ring, possessing following characteristics.
(i) Planarity
(ii) Complete de-localization of the π electrons in the ring
(iii) Presence of (4n + 2) π electrons in the ring
where n is an integer (n = 0, 1, 2, . . .). This is often referred to as Hückel Rule.
Some examples of aromatic compounds are given below:
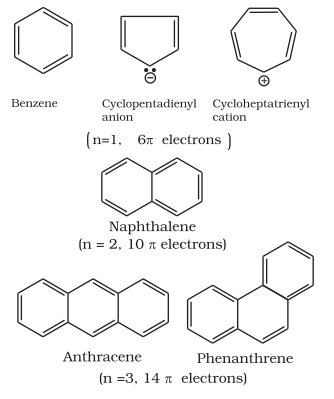
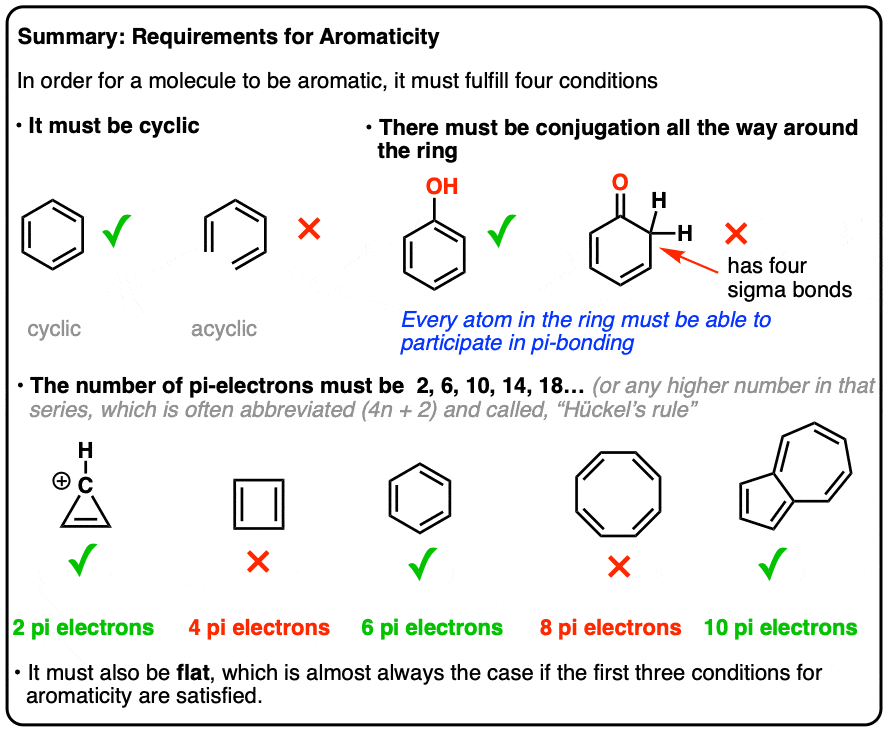
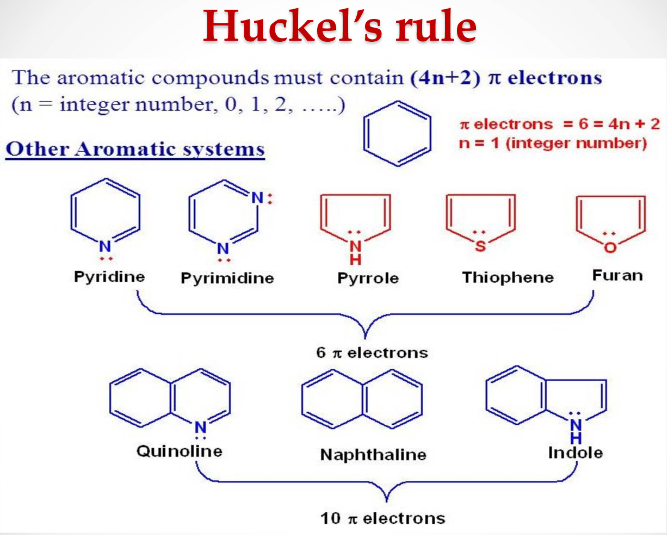
Click to rate this post!
[Total: 1 Average: 5]