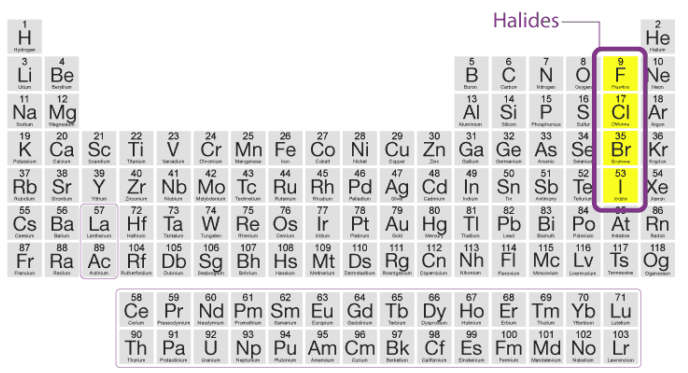
The alkali metal halides, MX, (X=F,Cl,Br,I) are all high melting, colourless crystalline solids. They can be prepared by the reaction of the appropriate oxide, hydroxide or carbonate with aqueous hydrohalic acid (HX). All of these halides have high negative enthalpies of formation; the ∆fH(-) values for fluorides
become less negative as we go down the group, whilst the reverse is true for ∆fH(-) for chlorides,
bromides and iodides. For a given metal ∆fH(-) always becomes less negative from fluoride to iodide.
The melting and boiling points always follow the trend: fluoride > chloride > bromide iodide. All these halides are soluble in water. The low solubility of LiF in water is due to its high lattice enthalpy whereas the low solubility of CsI is due to smaller hydration enthalpy of its two ions. Other halides of lithium are soluble in ethanol, acetone and ethyl acetate; LiCl is soluble in pyridine also.