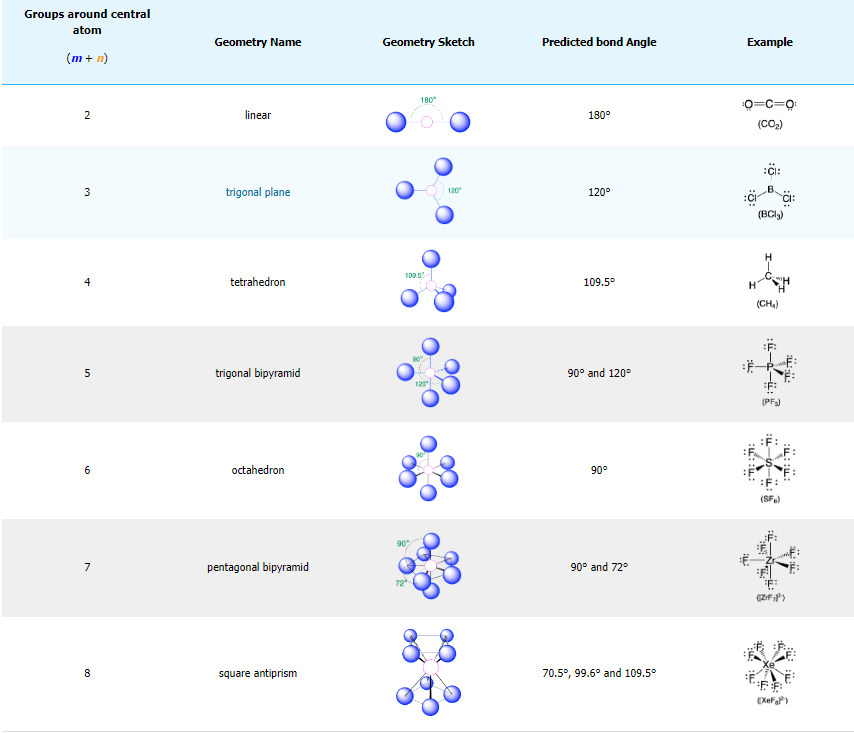
The Valence Shell Electron Pair Repulsion (VSEPR) Theory is a model used to predict 3-D molecular geometry based on the number of valence shell electron bond pairs among the atoms in a molecule or ion. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm.
The main idea of VSEPR theory is that pairs of electrons (in bonds and in lone pairs) repel each other.


Because electrons repel each other electrostatically, the most stable arrangement of electron groups (i.e., the one with the lowest energy) is the one that minimizes repulsion. Here, The pairs of electrons (in bonds and in lone pairs) are called “groups”. Groups are positioned around the central atom in a way that produces the molecular structure with the lowest energy.
The structures are: linear, trigonal planar, angled, tetrahedral, trigonal pyramidal, trigonal bipyramidal, disphenoidal (seesaw), t-shaped, octahedral, square pyramidal, square planar, and pentagonal bipyramidal.